 |
| advertisement: compare things at compare-stuff.com! |
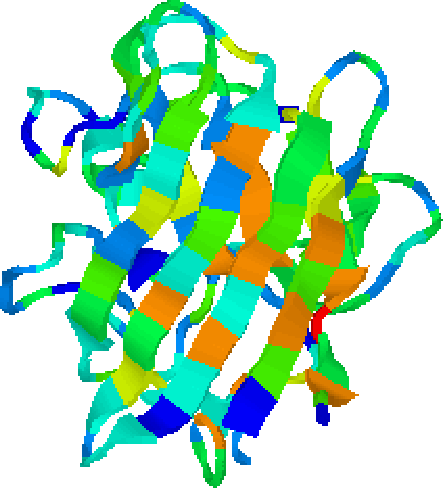
The main driving force of protein folding is the so-called hydrophobic collapse[Dill, 1990], resulting in a core of non-polar amino-acids whose side-chains pack against each other in a strongly hydrophobic environment. In experimentally derived protein structures this core is usually clearly defined. In particular, alternating patterns of hydrophobic and hydrophilic amino-acids (termed amphipathic) are evident in surface exposed strands and helices (Figure 5.7). The prediction and analysis of protein structure requires the separation of signal from noise in both sequences and structures. In this context, signal refers to conserved features across large evolutionary distances. Figure 5.7 also highlights the noise problem: there are alternating patterns of hydrophobicity in loop regions too. Through the use of self-organising maps we aim to automatically locate structurally important patterns in sequence properties such as hydrophobicity.
 |